Abstract
Background: Persistence or re-emergence of measurable residual disease (MRD) after remission induction therapy in B-cell acute lymphoblastic leukemia (B-ALL) is strongly associated with shorter relapse-free survival (RFS) and overall survival (OS). Inotuzumab ozogamicin (INO) is an anti-CD22 antibody-drug conjugate with the potential to eradicate MRD in patients (pts) with B-ALL, including in pts with prior exposure to blinatumomab.
Methods: This is a single-arm phase II trial of pts with B-ALL in complete remission (CR) who did not achieve MRD negativity (MRD-ve) or had MRD-positive (MRD+ve) relapse after at least 3 months (mos) from the start of frontline therapy or 1 month from the start of any salvage therapy. Eligibility was defined by MRD+ve at ≥ 0.01% by multiparametric flow cytometry (MFC) for Philadelphia chromosome (Ph) negative (Ph-) B-ALL and ≥ 0.01% by MFC and/or BCR::ABL1 transcripts for Ph positive (Ph+) B-ALL. MRD-ve was defined as undetectable MRD by MFC at a minimum sensitivity of 10-4 for Ph- B-ALL and undetectable MRD by both MFC and BCR::ABL1 transcripts at 10-4 for Ph+ B-ALL. For pts with p210 BCR::ABL1 transcript, the international scale (IS) was used for reporting. INO was administered at a dose of 0.6 mg/m2 on D1 and 0.3 mg/m2 on D8 of cycle 1 and 0.3 mg/m2 on D1 and D8 of cycles 2-6 (cycles given every 21-28 days). Pts received ursodiol prophylaxis while on INO. Pts with Ph+ ALL could receive a concomitant BCR::ABL1 tyrosine kinase inhibitor (TKI).
Results: Between 11/2018 and 6/2022, 27 pts with MRD+ve B-ALL were treated. The median age was 44 years (range, 19-70). Seventeen pts (63%) had Ph+ ALL (12 pts with p190 transcript and 5 pts with p210 transcript), 15 of whom received concurrent ponatinib and 2 dasatinib. Ten pts were Ph- (including 3 with Ph-like ALL). Twenty pts (74%) were in CR1, and 7 pts (26%) were in ≥ CR2 (4 in CR2, 2 in CR3 and 1 in CR4). Fourteen pts (52%) had received prior blinatumomab and 5 pts (18%) had prior stem cell transplantation (SCT). The median level of BCR::ABL1 by PCR in pts with Ph+ ALL was 0.21% (range, 0-18.9%) and median level by MFC in pts with Ph- ALL was 0.21% (range, 0.05-1.24%). Seven pts with Ph+ ALL were MRD+ve by MFC at a median of 0.04%, and 1 pt with Ph+ B-ALL was MRD+ve by MFC (1.15%%) but negative by PCR.
Eighteen pts (67%) responded and became MRD-ve, 16 after cycle 1 and 2 after cycle 2. In the Ph+ group, 10 pts (59%) responded; another 3 of 5 pts who had >0.1% BCR::ABL1 transcripts at enrollment attained major molecular response as best response. Eight of 10 (80%) pts with Ph- ALL responded. The median number of cycles was 3 (range, 1-6). Eleven of 13 pts (85%) without prior blinatumomab exposure became MRD-ve compared with 7/14 (50%) pts with prior blinatumomab exposure (p=0.1).
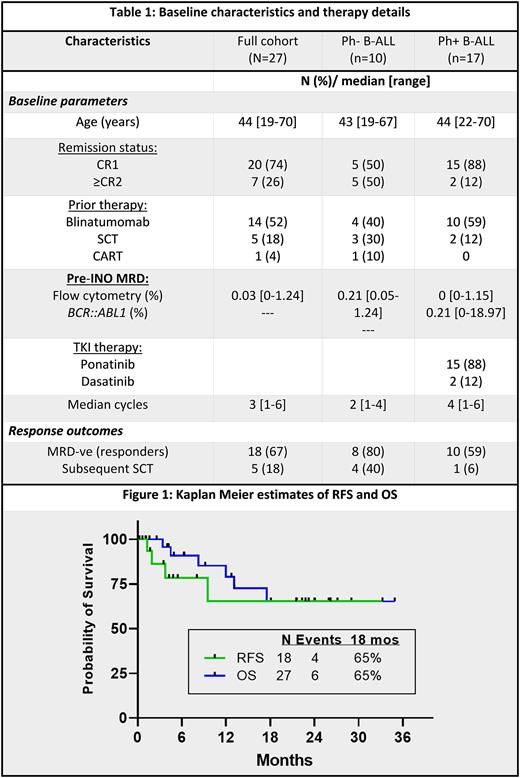
At a median follow up of 18 months (mos) (range, 1.1- 35 mos), 14 of the 18-responding pts (78%) are in ongoing MRD-ve CR. The median RFS and OS of the pts were not reached. The estimated 18-mos RFS and OS were 56% and 65%, respectively, for the whole group. 18-mos OS was 73% and 60% for responders and non-responders, respectively (p=0.7). Amongst the 18 responders, 5 pts were consolidated with SCT after a median of 3 cycles (range, 1-3) of INO, all in MRD-ve CR prior to SCT. Four (22%) responders had morphological relapse (including one relapse after SCT and one isolated CNS relapse). One pt died in MRD-ve CR due to SCT-related complications. Survival was similar between pts with Ph+ ALL vs. Ph- ALL (p=0.41), pts in CR1 vs. ≥ CR2 (p=0.23) and those with prior blinatumomab exposure vs. no prior exposure (p=40).
INO was overall well-tolerated with no grade 4 non-hematological toxicities. Two pts (7%) developed veno-occlusive disease (VOD); both had Ph+ ALL and were on concurrent ponatinib. One pt developed VOD (diagnosed radiologically) 1 month after ponatinib dose of 15mg/day) and another pt developed VOD (diagnosed by liver biopsy) during cycle 5 (cumulative INO dose of 3.3 mg/m2, ponatinib dose of 15mg/day). The overall 60-day mortality was 0%.
Conclusion: Lower doses of fractionated INO is a well-tolerated option for MRD eradication in pts with Ph+ or Ph- B-ALL, including in those with prior blinatumomab exposure or SCT. This approach can also facilitate consolidative SCT in these pts. The therapy is well tolerated, but pts should be monitored for development of VOD of the liver, though the absolute risks remain low.
Disclosures
Short:AstraZeneca: Consultancy; Astellas: Research Funding; Takeda Oncology: Consultancy, Research Funding; Pfizer: Consultancy; Novartis: Consultancy; Amgen: Consultancy, Honoraria; Stemline Therapeutics: Research Funding. Alvarado:FibroGen: Research Funding; Jazz Pharmaceuticals: Research Funding; Sun Pharma: Research Funding; Astex Pharmaceuticals: Research Funding; Daiichi-Sankyo/Lilly: Research Funding; BerGenBio: Research Funding. Burger:Novartis: Honoraria, Other: Travel, Accommodations, Expenses; Pharmacyclics LLC: Consultancy, Honoraria, Other: Travel, Accommodations, Expenses, Research Funding, Speakers Bureau; TG Therapeutics: Consultancy, Honoraria, Other: Travel, Accommodations, Expenses, Speakers Bureau; Gilead: Consultancy, Honoraria, Other: Travel, Accommodations, Expenses, Speakers Bureau; AstraZeneca: Research Funding; BeiGene: Consultancy, Research Funding, Speakers Bureau; Janssen: Consultancy, Honoraria, Other: Travel, Accommodations, Expenses. Jain:Genentech, Inc.: Consultancy, Honoraria, Other: Travel Support, Research Funding; Aprea Therapeutics: Research Funding; Cellectis: Honoraria, Research Funding; Adaptive Biotechnologies: Consultancy, Honoraria, Other: Travel Support, Research Funding; Kite, a Gilead Company: Consultancy, Honoraria, Research Funding; Dialectic Therapeutics: Research Funding; Novalgen: Research Funding; TransThera Sciences: Research Funding; Newave: Research Funding; Beigene: Honoraria; MEI Pharma: Honoraria; Cellectis: Honoraria, Research Funding; TG Therapeutics: Honoraria; Ipsen: Honoraria; CareDx: Honoraria; AstraZeneca: Consultancy, Honoraria, Other: Travel Support, Research Funding; Incyte Corporation: Research Funding; Pfizer: Research Funding; AbbVie: Consultancy, Honoraria, Other: Travel Support, Research Funding; Precision Biosciences: Consultancy, Honoraria, Other: Travel Support, Research Funding; Servier Pharmaceuticals LLC: Research Funding; ADC Therapeutics: Research Funding; BMS: Consultancy, Honoraria, Other: Travel Support, Research Funding; Takeda: Research Funding; Medisix: Research Funding; Loxo Oncology: Research Funding; Pharmacyclics, Inc.: Consultancy, Honoraria, Other: Travel Support, Research Funding; Janssen Pharmaceuticals, Inc.: Consultancy, Honoraria, Other: Travel Support; Mingsight: Research Funding; Fate Therapeutics: Research Funding. Konopleva:Stemline Therapeutics, F. Hoffman La-Roche; Janssen: Membership on an entity's Board of Directors or advisory committees; Reata Pharmaceuticals, Novartis and Eli Lilly: Patents & Royalties; Forty-Seven; F. Hoffman LaRoche: Honoraria; AbbVie, Genentech, F. Hoffman La-Roche, Eli Lilly, Cellectis, Calithera, Ablynx, Stemline Therapeutics, Agios, Ascentage, Astra Zeneca; Rafael Pharmaceutical; Sanofi, Forty-Seven: Research Funding; Stocks, Reata Pharmaceuticals: Current equity holder in publicly-traded company; AbbVie, Genentech, F. Hoffman La-Roche, Stemline Therapeutics, Amgen, Forty-Seven, Kisoji; Janssen: Consultancy. Ravandi:Astex/Taiho: Membership on an entity's Board of Directors or advisory committees, Research Funding; BMS/Celgene: Consultancy, Honoraria, Research Funding; Amgen: Honoraria, Research Funding; Syos: Consultancy, Honoraria, Research Funding; Novartis: Consultancy; Abbvie: Consultancy, Honoraria, Research Funding; Prelude: Research Funding; Xencor: Research Funding; AstraZeneca: Consultancy; Astellas: Consultancy, Honoraria, Research Funding; Biomea Fusion, Inc.: Research Funding; Amgen: Honoraria, Research Funding. DiNardo:LOXO: Research Funding; Kura: Honoraria, Membership on an entity's Board of Directors or advisory committees; ImmuneOnc: Honoraria, Research Funding; Bluebird Bio: Honoraria; Bristol Myers Squibb: Honoraria, Research Funding; Foghorn: Honoraria, Research Funding; AbbVie: Consultancy, Research Funding; Astellas: Honoraria; Servier: Consultancy, Honoraria, Research Funding; Jazz: Honoraria; Notable Labs: Current holder of stock options in a privately-held company, Membership on an entity's Board of Directors or advisory committees; Novartis: Honoraria; Takeda: Honoraria; Astex: Research Funding; GlaxoSmithKline: Membership on an entity's Board of Directors or advisory committees; GenMab: Membership on an entity's Board of Directors or advisory committees; Cleave: Research Funding; Forma: Research Funding; Gilead: Honoraria. Sasaki:Otsuka Pharmaceuticals: Honoraria; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Daiichi-Sankyo: Membership on an entity's Board of Directors or advisory committees; Pfizer: Membership on an entity's Board of Directors or advisory committees. Thompson:AbbVie, Pharmacyclics, Adaptive Biotechnologies, Genentech: Research Funding; Adaptive Biotechnologies: Membership on an entity's Board of Directors or advisory committees; AbbVie, Gilead, Janssen, Pharmacyclics, Adaptive Biotechnologies, Genentech: Consultancy; AbbVie, Gilead, Janssen, Pharmacyclics, Adaptive Biotechnologies, Genentech, Amgen: Honoraria. Ferrajoli:AstraZeneca: Membership on an entity's Board of Directors or advisory committees, Research Funding; Beigene: Research Funding; Janssen: Membership on an entity's Board of Directors or advisory committees. Kantarjian:Pfizer: Honoraria, Research Funding; ImmunoGen: Research Funding; AbbVie: Honoraria, Research Funding; Amgen: Honoraria, Research Funding; Ascentage: Membership on an entity's Board of Directors or advisory committees, Research Funding; Astellas Health: Honoraria, Membership on an entity's Board of Directors or advisory committees; Daiichi-Sankyo: Consultancy, Research Funding; KAHR Medical Ltd: Honoraria, Membership on an entity's Board of Directors or advisory committees; Novartis: Honoraria, Research Funding; NOVA Research: Honoraria; Jazz Pharmaceuticals: Research Funding; Ipsen Pharmaceuticals: Honoraria, Membership on an entity's Board of Directors or advisory committees; Takeda: Honoraria. Jabbour:Bristol Myers Squibb: Other: Advisory Role, Research Funding; Takeda: Other: Advisory Role, Research Funding; AbbVie: Other: Advisory Role, Research Funding; Spectrum: Research Funding; Pfizer: Other: Advisory Role, Research Funding; Genentech: Other: Advisory Role, Research Funding; Amgen: Other: Advisory Role, Research Funding; Adaptive Biotechnologies: Other: Advisory Role, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal